Vanadium
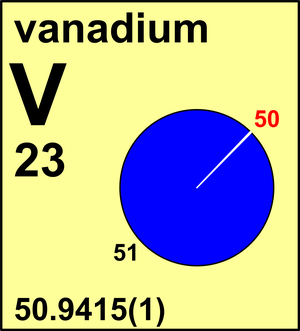
| Isotope | Atomic mass (Da) | Isotopic abundance (amount fraction) |
|---|---|---|
| 50V | 49.947 156(3) | 0.002 50(10) |
| 51V | 50.943 957(3) | 0.997 50(10) |
In its 1961 report, the Commission accepted Ar(V) = 50.942 based on mass-spectrometric data, and in 1969 recommended a more precise Ar(V) = 50.9414(3). A number of determinations of the isotopic composition of vanadium have since been considered. As a result, in 1977 the Commission refined Ar(V) to 50.9415(1). The isotopic composition of vanadium from five chondritic meteorites has been shown identical within experimental error to the terrestrial diabase W-1 and a laboratory standard.
Two stable isobars, 50Ti and 50Cr, are the immediate neighbours in the chart of nuclides, to 50V, whose β+ and β- decay modes are, therefore, predictable. The nuclear angular momentum of 50V, however, is high but consistent with long half-lives, evidently too long to be readily observed. The isobars render the mass-spectrometric determination of the abundance of the isotope 50V subject to careful chemical determination of the trace presence of Ti and Cr.
© IUPAC 2003
CIAAW
Vanadium
Ar(V) = 50.9415(1) since 1977
The name derives from the Scandinavian goddess of love and beauty, Freyja Vanadis, because of its
many beautiful multi-coloured compounds. Vanadium was discovered by the Swedish physician and chemist
Nils-Gabriel Sefström in 1830.
Vanadium had originally been discovered by the Spanish mineralogist Andres Manuel
del Rio y Fernandez in 1801, who named it erythronium, after the plant of that name whose flowers
have many beautiful colours. Del Rio later decided that it was really chromium in his lead sample.
Vanadium metal was first isolated by the English chemist Henry Enfield Roscoe in 1869.


